Drug users for weight loss may be found to be climbed.
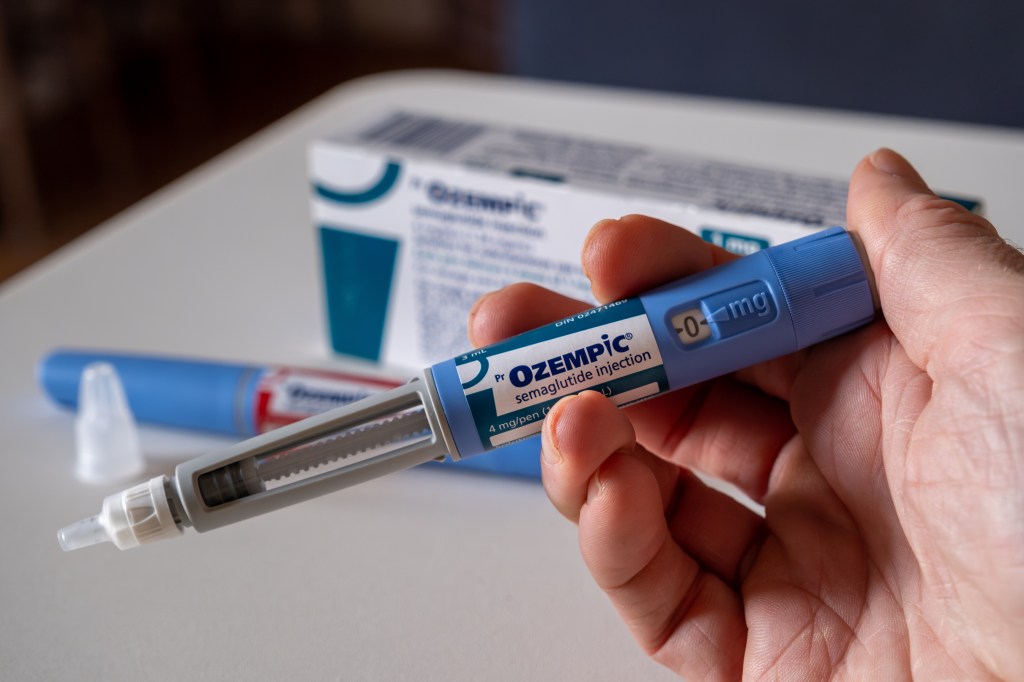
Food and drug administration is being reduced to copycat versions of Ozempic (the name of the semaglutide brand) and Zepbound) the name of the Tirzepatide brand, threatening a solution that allowed patients to access more cheaper alternatives during shortage.
Compound pharmacies had been producing these out -of -brand versions under special bonuses due to the scarcity of the FDAs approved.
Since the FDA declared scarcity, these flexibilities are revoked, which caused the medicine compounds and telemedicine companies to move to find solutions to continue sales.
The period of grace to produce and sell a composite tirzepatide ended in March. Thursday was the final cut of the composite semaglutide.
These drugs mimic GLP-1-the Hormone that the body naturally produces after eating, so that users feel more full for longer.
The association of outsourcing facilities, a commercial group representing composite pharmacies, challenged the FDA’s decision in court, but the judges confirmed the authority of the agency to enforce the ban.
This movement is expected to premiere the supply of these medicines, which may lead to higher costs for patients who have trusted in the most affordable composite versions.
“ Patients who can get the drug composed today for $ 350 a month will have no option to pay $ 1,000 a month for Mounjaro or Zepbound, large brand names of Pharma’s name that insurance will not normally not cover, if they can find it, that is, ” wrote the former Secretary of the White House Press, Sean Spicer, at the scene.
“In a nutshell, Tirzepatide will become inaccessible during the night for many who trust him.”

Spicer, 53, said that he “tried all weight loss medicines” and that “GLP-1 have really been a game changing room”, which is why he believes that this ban would greatly affect him and millions of other Americans who have depended on budget-favorable drugs.
“More than 12% of American adults have used GLP-1 drugs to manage chronic conditions, such as diabetes, heart disease and obesity,” said Spicer, who receives a telemedicine company. “A triplicate of their costs will be devastating for those who have come to trust -“
The Wall Street Journal this week reported that composite pharmacies and Telehealth companies have slightly modified doses, added vitamins or changed how medicines are administered to continue selling.
They claim that the law allows personalized medicines, an interpretation that classifies pharmaceutical companies. The challenges are almost certain.
The repression of the FDA aims to address the security problems associated with composite medicines, which are not subject to the same process of rigorous approval as their brand counterparts.
Increasing Telehealth’s companies – which critics believe that they do not evaluate their patients enough or spread possible side effects – is added to concern.
However, many experts believe that these concerns are overcome by the health benefits of GPP-1, especially since the United States is in the midst of an obesity epidemic.
“The scarcity is much better; insurance coverage is much worse,” Dr. Disha Narang, an endocrinologist and director of Obesity Medicine in Endaavour Health, told CNN.
“From a practical point of view, patients cannot get benefits to medication, which now almost more than 50% of our country can be described.
#FDA #breaks #Ozempic #copycats #options #potentially #reduced #consumers
Image Source : nypost.com